Some of the most successful animals on earth live in societies characterised by a division of labour between reproducing and non-reproducing castes. One role non-reproducing members may undertake is defence. Spectacular examples include the heavily armoured termites and ants. Recently a soldier caste was discovered in an entirely new and unexpected battleground, inside the bodies of snails. The soldiers? Tiny parasitic flatworms.
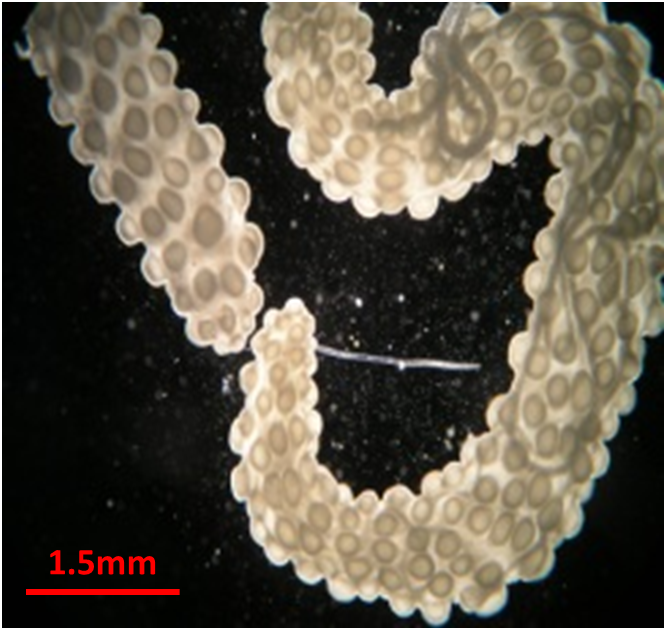
Flatworms, or trematodes, have complicated life cycles, involving several different stages infecting a variety of host species. In one host, often a snail, a single trematode undergoes repeated clonal reproduction. Clones produce more clones or go on to produce the next infective stage, which leaves the snail to infect the final host. While working with trematode colonies of Himasthla sp. infecting the Californian horn snail Cerithidea californica, researchers at the University California Santa Barbara observed that the trematode occurred in two distinct morphological forms. There was a large reproducing primary morph, which appeared to be the form typically described in the literature, and a secondary smaller, thinner morph.
These secondary morphs had a number of other features which set them apart. They rarely showed any signs of reproduction and were far more active. They also had huge muscular pharynxes and guts relative to their larger sisters. When researchers preformed behavioural tests, they discovered just what those large mouth parts were for. The secondary morphs attacked and killed other trematode species and unrelated conspecifics. This behaviour is not unknown in trematodes; a number of species attack and kill other trematodes. What was novel was that the smaller morphs appeared to be doing all attacking. The behaviour was rarely observed in the primary morphs. There was also a spatial segregation of morphs. Primary morphs were located in the visceral mass, mainly in the region of the gonads. The secondary morphs were more widely distributed though mainly found within the mantle. The snail mantle is the main entry point for trematodes, a strategic area to defend against invading armies. Finally, the researcher found very few intermediate morphs, suggesting that the smaller morphs were not simply juvenile stages of the primary morphs. They were a distinct, permanent caste whose function appeared to be defence – soldiers. The researchers had discovered eusociality in a completely new taxonomic group. Previously, eusocial systems consisting of morphologically distinct, specialised reproductive and non-reproductive castes had only been recognised in insects, snapping shrimp, a sea anemone and mole rats. The researchers have already suggested a further five species of trematodes that may have soldier castes.
Work from New Zealand, published this year, on another species (Philophthalmus sp.) has expanded the list of trematodes with soldier castes. The authors also showed that interspecific competition has a heavy impact on colony numbers. This is just the sort of pressure that favours adaptive strategies to reduce competition, such as a permanent soldier caste. However, competition may not be the only selective pressure driving or maintaining caste differentiation in trematodes. In the absence of competition, the presence non-reproducing morphs were found to provide a benefit to the colony, as measured by the number of infective stages produced. Precisely how this benefit comes about is not yet known. The authors suggest some form of communication or nutrient exchange may be taking place between the two morphs. This gives tantalising hints that these colonies are even more complex and interactive than previously thought.
Not only has the discovery of the eusociality in trematodes widened the taxonomic range of this phenomenon, it has also provided researchers with an exciting new tool to study its evolution. The Trematoda class contains at least 20, 000 species with a wide variety of life-histories and ecologies. The discovery is also a great example of how new and unexpected results can still come from well-studied animals. The Himasthla sp. /Californian horn snail system had been studied for over 65 years.
Author
Karen Loxton: loxtonk[at]tcd.ie
Photo credits
wikimedia commons